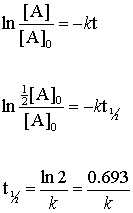half life formula chemistry
Using the concentration-time equation for a second-order reaction we can solve for half-life. We can determine that the order of a decay reaction is 1 by looking at the.
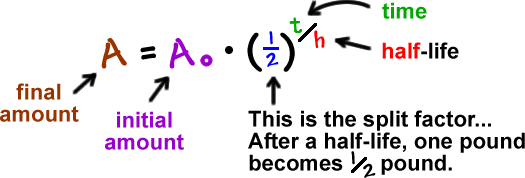
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels
So now you have after one half-life-- So lets ignore this.

. The half-life formula for chemistry is T ln2lambda. In order to calculate the half-life of a chemical specie integrated half-life equations are used according to the order of reactions. For a first zero order reaction the.
Given half life of the substance is t1 2 t 1 2 004. Find the value of the decay constant of a radioactive substance having a half-life of 004 seconds. N t is the remaining quantity of a substance after time t has elapsed.
Half life formula chemistry Saturday July 30 2022 Edit. Half-Life is the required time for a sample of radioactive material to reach half the mass of the original sample. The equations are given above.
N 0 is the. For a zero-order reaction the half-life equation is given as. T is the half-life and lambda is the decay constant which is specific to each chemical.
So we started with this. All 10 grams were carbon. What is a simple definition of.
Substitute this information into the equation for the half life of a reaction with this order and solve for t ½. N t N_0 times 05 tT N t N 0. To learn more about the half life formula of Zero first second nth reaction Visit.
For a zero-order reaction the mathematical expression that can be. Half-life Half-life thalf is defined as the amount of time required for the amount of a substance to be reduced by 50. Half-life a useful concept if its value does not depend on how much.
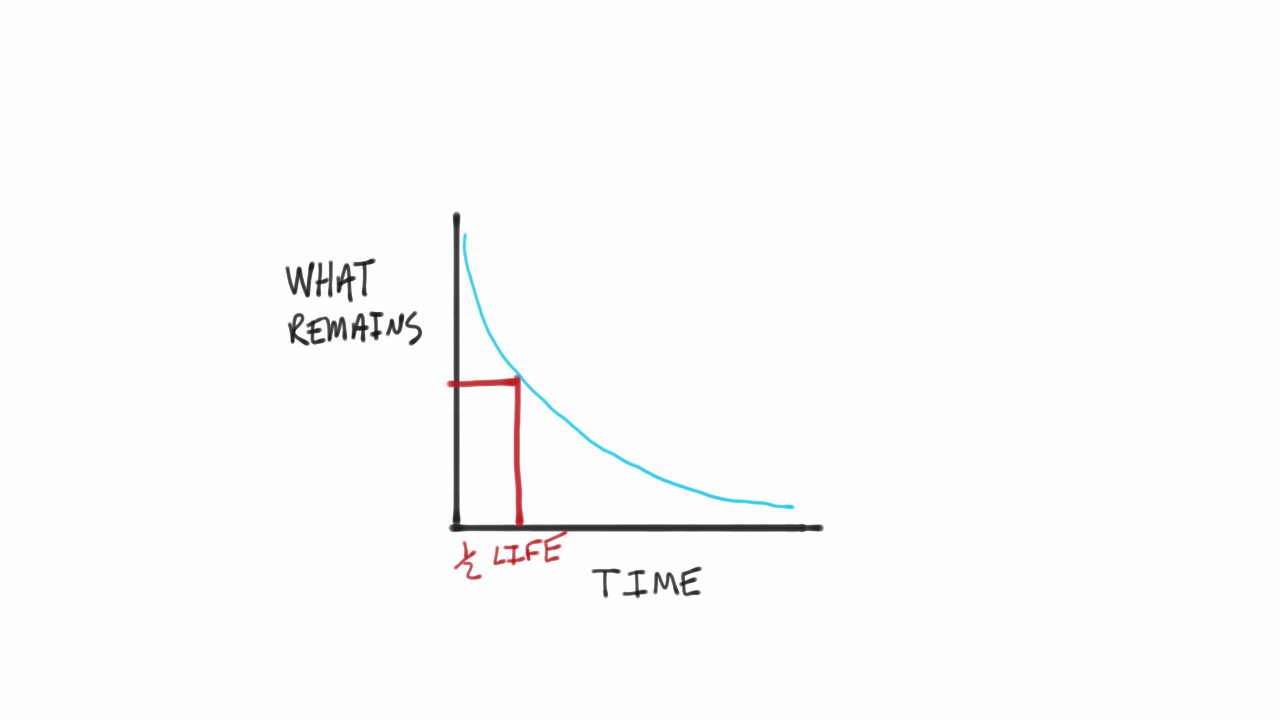
The time interval that is required for one-half of the atomic nuclei of a radioactive substance to disintegratedecay is known as the half-life of the substance. C-14 Half-Life 5730 Years. The half-life formula for a reaction depends upon the order of a reaction.
This expression works best when the number of half-lives is a whole number. In nuclear reactions this time period. Half life formula- The time taken for half of reactions to complete or the time at which the concentration of the reactant is reduced to half of its original value is called the half life period of the reaction.
We know that at the half-life time eqt_12 eq the concentration of the reactant will. We utilize the equation that relate amount remaining initial mass and number of half-livesn. N t N 0 05 t n.
T1 2 0693 λ t 1 2 0693 λ. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. It is important to note that the formula for the half-life of a reaction varies with the order of the reaction.
The half-life formula for various reactions is given below. N t 1 2 n X N o 1 2 4 X 50 3125 g. So if you go back after a half-life half of the atoms will now be nitrogen.
By using the following decay formula the number of unstable nuclei in a radioactive element left after t can be calculated. So the value of t can be found or determined using half life equation. What is the half.
Converting a Half Life to a Rate Constant.

Organic Chemistry Half Life And Shelf Life Of Second Order Reaction Chemistry Stack Exchange

How To Plot A Half Life Graph Chemistry Study Com
Half Life The Decay Of Knowledge And What To Do About It Farnam Street

Zero Order Reaction Definition Examples Formula
Half Life Introductory Chemistry
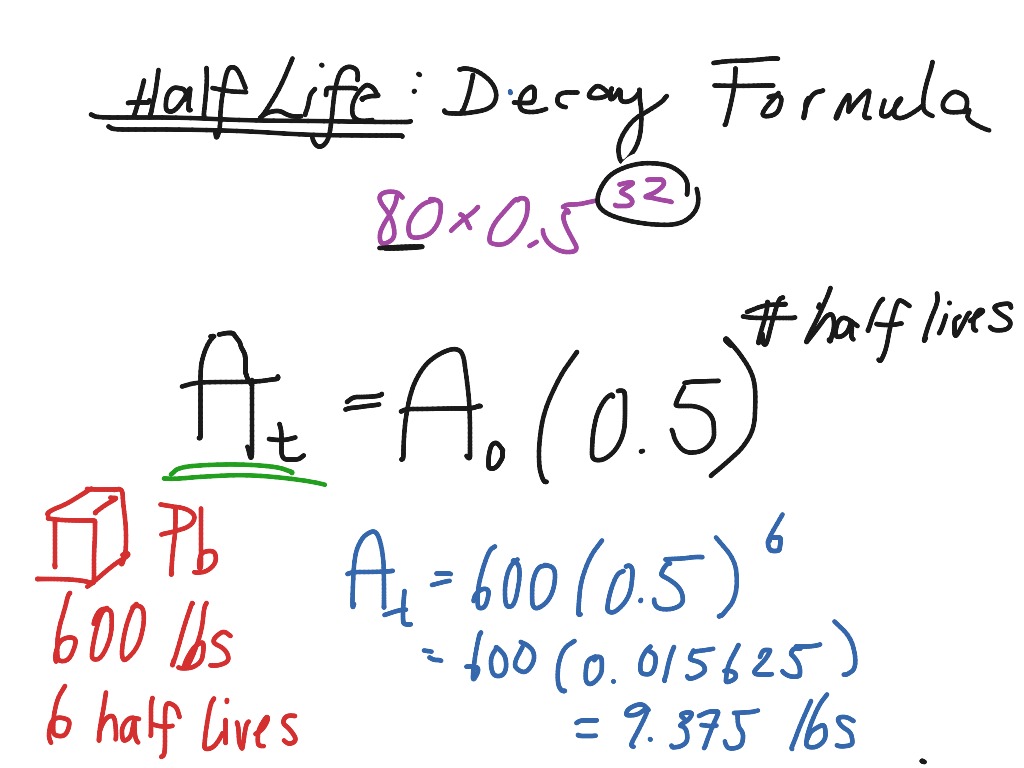
Radioactive Decay Formula Science Chemistry Nuclear Chemistry Showme

Half Life Of Zero Th 0th Order Reaction Derivation Youtube

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com
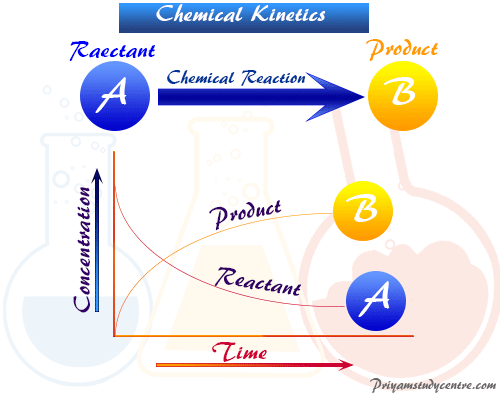
Chemical Kinetics Half Life Definition Formula Equations
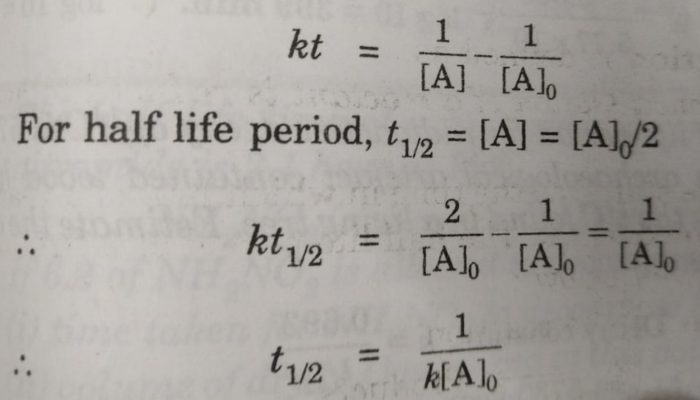
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
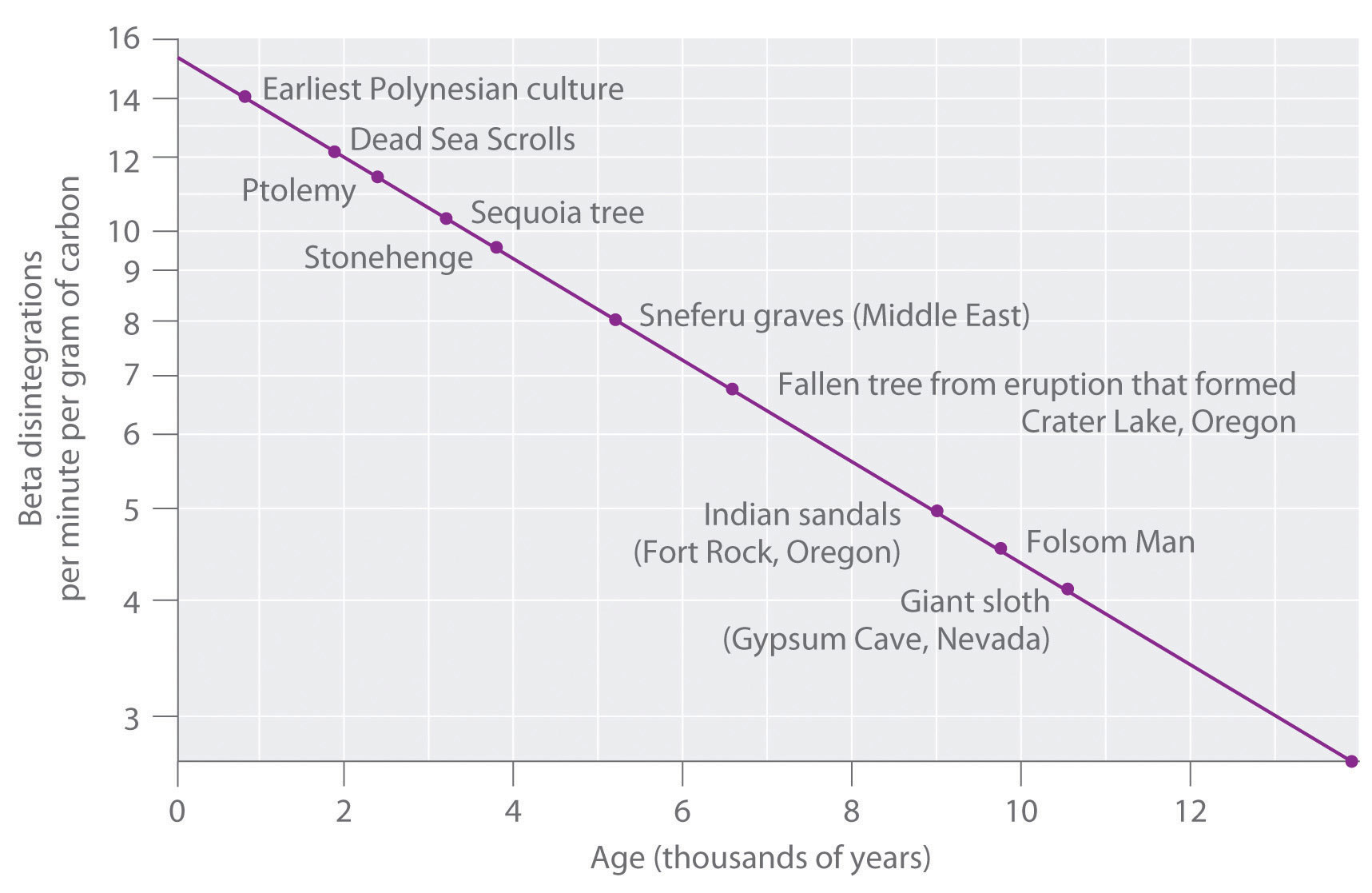
Half Lives And Radioactive Decay Kinetics Chemistry Libretexts
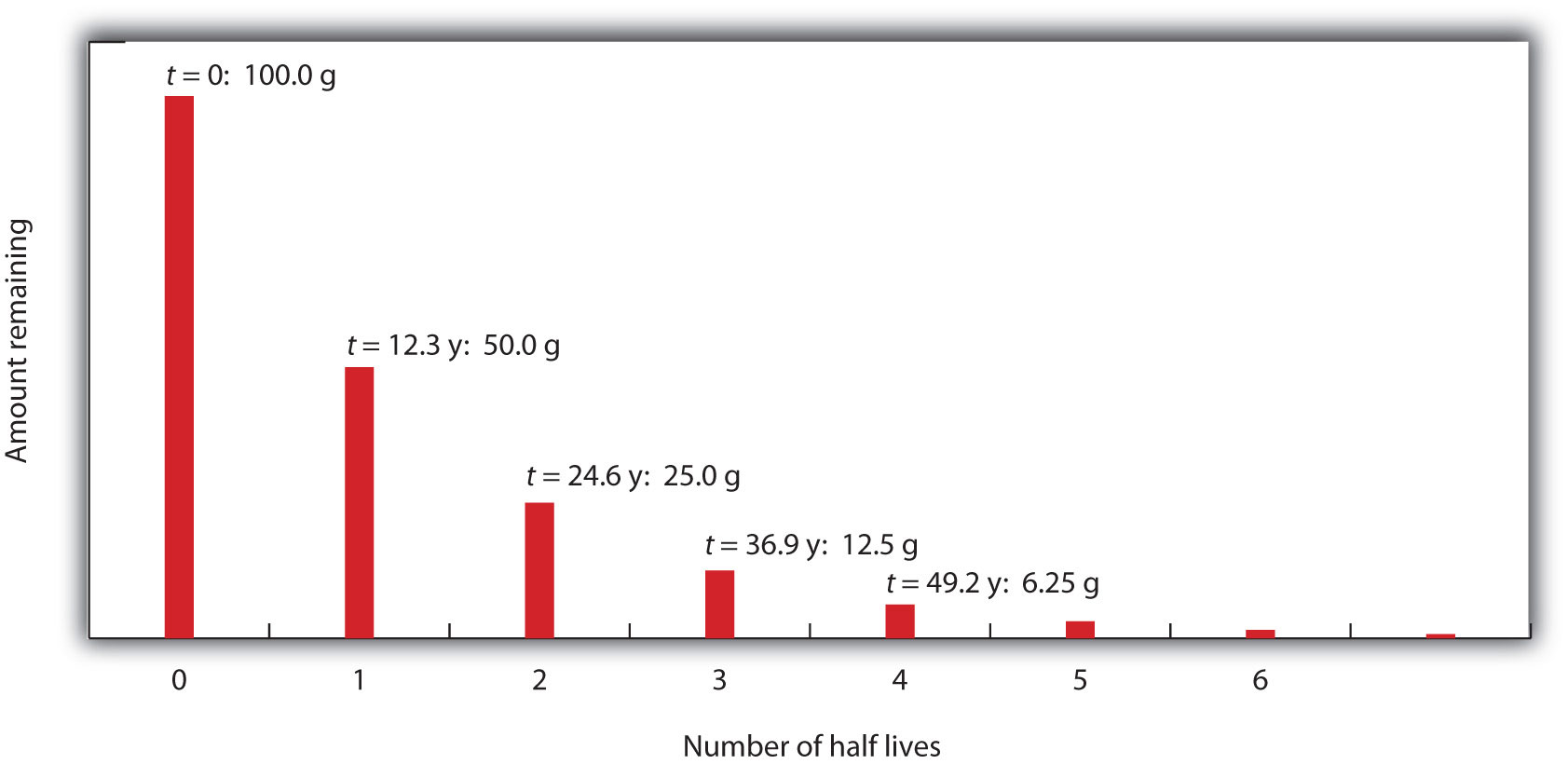
11 2 Half Life Chemistry Libretexts
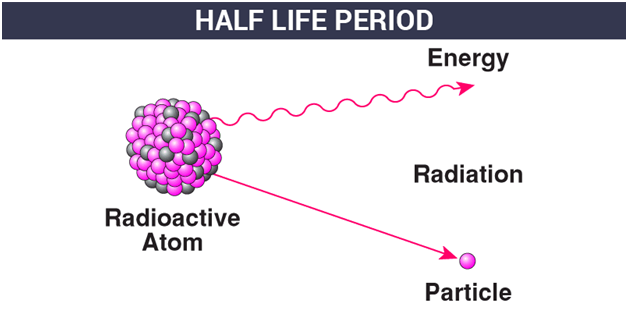
Half Life Period Radioactive Decay Mean Life Byju S
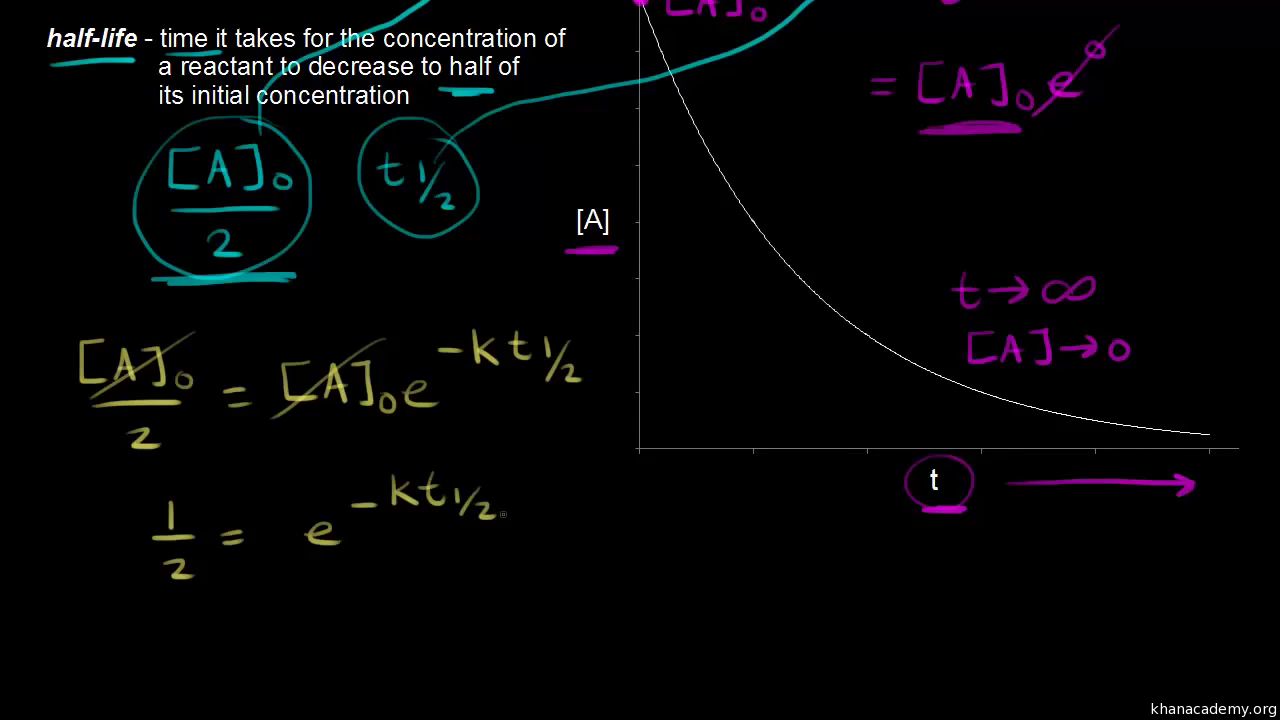
Half Life Of A First Order Reaction Video Khan Academy

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
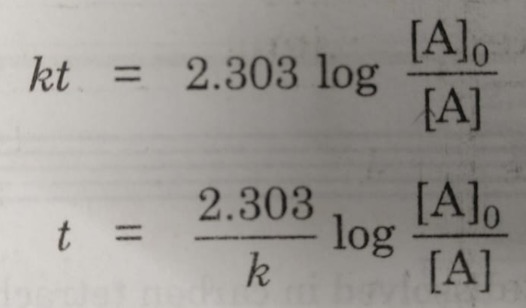
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12